Wayne Gillam

Center for Neurotechnology (CNT) members at the University of Washington (UW), in collaboration with NeuroRecovery Technologies, are developing a novel, non-invasive therapeutic approach for people with spinal cord injury, which promotes long-term recovery of hand and arm function.
On December 7, 2014, Joe Beatty, then 60 years old, was body-surfing on Kaanapali Beach in Hawaii, a popular place with tourists on the west side of Maui island and known for its calm, crystal-blue ocean waters. Long considered a family-friendly beach, Kaanapali is widely thought of as an ideal spot for both children and adults to swim; however, on that day for Beatty, this normally safe environment turned unusually dangerous.
“It was a rogue wave. It really was, that I caught and gave me a header in the sand. I was knocked out for three days,” Beatty said. “It happened on a Sunday, and I didn’t get totally back into consciousness until Wednesday. That’s when I first learned anything had happened. When they brought me to the hospital by ambulance, I was told that the only thing moving was my big toe, and my heart rate was down to nine beats a minute. I was lucky to be alive.”
…When they brought me to the hospital by ambulance, I was told that the only thing moving was my big toe, and my heart rate was down to nine beats a minute. I was lucky to be alive.
–CNT research study participant, Joe Beatty, describing effects of his spinal cord injury
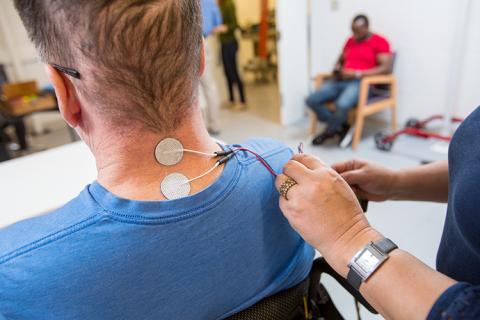
Acute magnetic resonance imaging revealed hemorrhage and contusion of Beatty’s cervical spine. Beatty was physically fit for most of his life and enjoyed hiking, swimming and other vigorous outdoor activities, but this injury left him with severe loss of motor control and sensation in all four of his limbs.
“My life has changed 100 percent,” Beatty said. “I can’t walk on my own. I can’t get around on my own. Even though I can move my arms a little, my fingers are sort of locked-up.”
Over the next two years, Beatty underwent a laminectomy to relieve pressure on his spinal cord and an arthrodesis to stabilize the bones of his spine. A side effect from his injury was dramatic weight loss. As muscles began to atrophy from lack of use over time, Beatty, who is six feet tall, saw his weight drop from 203 pounds before the accident to only 128 pounds. Rehabilitative work and physical therapy at the UW, Kline Galland Home and Pushing Boundaries helped Beatty recover precious muscle mass, but he still was completely dependent for all self-care activities, such as feeding, bathing, dressing and grooming. Although he experienced some limited improvements to motor control and sensation in his limbs after the accident, by late 2016 his recovery had reached a plateau.

Fortunately for Beatty, his son worked at Kline Galland Home and used resources there to contact CNT Co-Director, Chet Moritz. Beatty’s son met with Moritz and UW neurosurgeon and CNT member, Christoph Hofstetter, to find out if Beatty could qualify for a new study the CNT was starting in December 2016. Because Beatty’s injury was a contusion and not a complete severing of the spinal cord, he was an ideal participant.
Developing a new way to help the spinal cord adapt and recover after injury
Moritz and his team at the UW are working on a unique approach to spinal cord injury rehabilitation that combines physical therapy with non-invasive electrical stimulation to the spinal cord. The study Beatty is now participating in takes place in the UW AMP Lab and involves a standard physical therapy program that includes stretching, strengthening, active assistive range of motion exercises and intensive fine motor skill training tailored to Beatty’s functional needs. The rehabilitative regimen Moritz’ team is putting Beatty through also includes a technique called transcutaneous spinal stimulation. This relatively new approach to stimulating the spine uses electrical stimulation applied to the surface of the skin to activate neurons located within the spinal cord without discomfort for the participant.
The long-term benefits we’re seeing are one of the first clear examples of engineered plasticity in human subjects. We’re using a neural device to make long-term changes in neural circuits after spinal cord injury.
–CNT Co-Director, Chet Moritz

“It’s like a tiny shock, sort of like how you can get zapped by static electricity,” Beatty said, “but I’ve experienced static electricity even more painful than this, so it’s light, it’s nothing, especially when it’s ramped-up slowly so your body gets used to it.”
Transcutaneous spinal stimulation works using principles similar to epidural spinal stimulation, a rehabilitative method requiring implantation of a stimulation device, but because it is administered on the surface of the skin, transcutaneous spinal stimulation has the advantage of being non-invasive.
“Epidural stimulation electrodes are implanted during surgery and are located right on the surface of the spinal cord, so they require little current to activate the spinal cord,” Moritz explained. “With transcutaneous stimulation, we use a wave form that numbs the skin, so that the higher-current stimulation can pass through the skin without causing pain and activate the spinal cord below.”
While the stimulator is on, it acts like a hearing-aid for the spinal cord, making neurons in the spinal cord more receptive and able to “hear” signals from the brain. Descending signals from the brain, which normally would not be able to cause muscle movement in a person like Beatty, are able to cause muscles to contract and movement to happen. Participants are then able to exert greater force in their upper body and make movements that they would not ordinarily be able to do.

When stimulation is combined with physical therapy, the results are dramatic. Beatty’s upper extremity muscle strength has nearly doubled over the course of the study and stabilized at 75% stronger than baseline measurements, even for three months without further treatment. Starting from the very first stimulation session, almost all motor functions of Beatty’s hands and arms improved, and he now feels substantial increases in upper-body sensation. What this means in real-life terms for Beatty is greater independence.
“It used to be that Austin [Beatty’s caregiver] would have to get up and give me my pills at night. Now, the pills are just on my chest, so when I wake-up, I can take the pills myself,” Beatty said. “I have a backpacking bag that I use for water, so I have access at night to liquids too. I do that all myself now.”
Remarkably, the study has been showing that participants can see benefits for weeks or even months after the stimulator is turned off. This indicates that the brain and spinal cord use the stimulation experience to adapt, and that the effects of transcutaneous spinal stimulation when combined with physical therapy are both immediate and long-lasting.
“The long-term benefits we’re seeing are one of the first clear examples of engineered plasticity in human subjects,” Moritz said. “We’re using a neural device to make long-term changes in neural circuits after spinal cord injury.”
Next steps
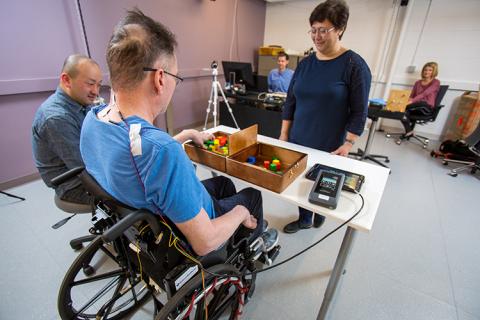
The CNT’s industry affiliate, NeuroRecovery Technologies, supplied the stimulator to the research team, as well as guidance and training on how to use it. In addition to the already-existing study happening at the CNT, the company is currently preparing to spin-up four other research sites to be located in Colorado, Illinois, Minnesota and New Jersey for a multi-site clinical trial. Establishing sites in these locations will help give patients across the country easier access to a research facility. Sites participating in the clinical trials will also use the CNT’s research approach (physical therapy combined with transcutaneous spinal stimulation) to reproduce clinical applications of this therapeutic technique.
The safety study will hopefully begin within the next year, and if all goes well, the stimulator could be available to patients immediately, which is a much faster pathway than an implanted device. That’s one of the reasons we’re excited about this treatment, because we can get it out to the community of people with spinal cord injury soon.
–CNT Co-Director, Chet Moritz
The CNT is now recruiting more participants with challenges using their hands and arms after spinal cord injury, and the center will continue to test this novel form of stimulation as an innovative approach to improving hand and arm function. Because the treatment method is both non-invasive and low-risk to the patient, the turnaround time from the lab to clinical application is shorter than it would be for most devices that are surgically implanted.
“The safety study will hopefully begin within the next year, and if all goes well, the stimulator could be available to patients immediately, which is a much faster pathway than an implanted device,” Moritz said. “That’s one of the reasons we’re excited about this treatment, because we can get it out to the community of people with spinal cord injury soon.”
From Beatty’s perspective, having already experienced benefits from participating in the study, he’s determined to continue his involvement. He is looking forward to what this technology, therapeutic approach and future research findings will bring not only for himself, but for others with spinal cord injury as well.
“I can see gradual improvements. Like everybody else, I wish it would go faster, but just have faith and hang in there, because one day, hopefully, there will be a breakthrough,” Beatty said. “When it happens, I want to be the first one. If I can do it, it’s going to make a difference.”
